ಇತ್ತೀಚೆಗೆ, ಜಿಯಾಕ್ಸಿಂಗ್ ಮಾರುಕಟ್ಟೆ ಮೇಲ್ವಿಚಾರಣಾ ಆಡಳಿತವು ಹೈಯಾನ್ ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯ ಉಪಕರಣ ಕಂಪನಿ, ಲಿಮಿಟೆಡ್ನ ಪ್ರಕ್ರಿಯೆ ನೀರಿನ ಸಮಗ್ರ ಮಾದರಿಯನ್ನು ನಡೆಸಿತು ಮತ್ತು ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯದ ಪ್ರಕ್ರಿಯೆ ನೀರು 2020 ರ ಆವೃತ್ತಿಯ ಚೈನೀಸ್ ಫಾರ್ಮಾಕೊಪೊಯಿಯಾದ ಶುದ್ಧೀಕರಿಸಿದ ನೀರಿನ ಅವಶ್ಯಕತೆಗಳಿಗೆ ಸಂಪೂರ್ಣವಾಗಿ ಅನುಗುಣವಾಗಿದೆ ಎಂದು ಘೋಷಿಸಿತು, ಇದು ಉತ್ಪನ್ನದ ಗುಣಮಟ್ಟ ಮತ್ತು ರೋಗಿಗಳ ಸುರಕ್ಷತೆಯಲ್ಲಿ ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯದ ಅತ್ಯುತ್ತಮ ನಿಯಂತ್ರಣ ಸಾಮರ್ಥ್ಯವನ್ನು ಮೌಲ್ಯೀಕರಿಸುತ್ತದೆ.
ಮಾದರಿ ಪರಿಶೀಲನೆಯನ್ನು ಜಿಯಾಕ್ಸಿಂಗ್ ಮಾರುಕಟ್ಟೆ ಮೇಲ್ವಿಚಾರಣಾ ಆಡಳಿತವು ಆಯೋಜಿಸಿದ್ದು, ಜಿಯಾಕ್ಸಿಂಗ್ ಆಹಾರ, ಔಷಧ ಮತ್ತು ಉತ್ಪನ್ನ ಗುಣಮಟ್ಟ ತಪಾಸಣೆ ಮತ್ತು ಪರೀಕ್ಷಾ ಸಂಸ್ಥೆಯಿಂದ ನಿಯೋಜಿಸಲಾಗಿದೆ. ಸಂಬಂಧಿತ ರಾಷ್ಟ್ರೀಯ ಮಾನದಂಡಗಳು ಮತ್ತು ಉದ್ಯಮದ ಮಾನದಂಡಗಳಿಗೆ ಅನುಗುಣವಾಗಿ, ತಪಾಸಣೆ ಮತ್ತು ಪರೀಕ್ಷಾ ಸಂಸ್ಥೆಯು ನೀರಿನ pH, ನೈಟ್ರೇಟ್, ವಾಹಕತೆ, ಭಾರ ಲೋಹಗಳು, ಸೂಕ್ಷ್ಮಜೀವಿಯ ಮಿತಿಗಳು ಮತ್ತು ಇತರ ಹಲವು ಅಂಶಗಳನ್ನು ಒಳಗೊಂಡಂತೆ ವಿವಿಧ ವೈದ್ಯಕೀಯ ಸಾಧನಗಳನ್ನು ಉತ್ಪಾದಿಸಲು ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯ ಬಳಸುವ ಪ್ರಕ್ರಿಯೆ ನೀರಿನ ಕುರಿತು ಸಮಗ್ರ ಮತ್ತು ವೃತ್ತಿಪರ ಪರೀಕ್ಷೆಯನ್ನು ನಡೆಸಿದೆ. ಹಲವಾರು ಸುತ್ತಿನ ಕಠಿಣ ಪರೀಕ್ಷೆಯ ನಂತರ, ಫಲಿತಾಂಶಗಳು ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯದ ಪ್ರಕ್ರಿಯೆ ನೀರು 2020 ರ ಆವೃತ್ತಿಯ ಚೀನೀ ಫಾರ್ಮಾಕೊಪೊಯಿಯಾದ ಶುದ್ಧೀಕರಿಸಿದ ನೀರಿನ ಅವಶ್ಯಕತೆಗಳನ್ನು ಸಂಪೂರ್ಣವಾಗಿ ಪೂರೈಸುತ್ತದೆ ಎಂದು ತೋರಿಸುತ್ತದೆ, ಇದು ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯ ಸಾಧನ ಉತ್ಪನ್ನಗಳ ಗುಣಮಟ್ಟದ ಸುರಕ್ಷತೆ ಮತ್ತು ವಿಶ್ವಾಸಾರ್ಹತೆಯನ್ನು ಸಂಪೂರ್ಣವಾಗಿ ಖಾತರಿಪಡಿಸುತ್ತದೆ.
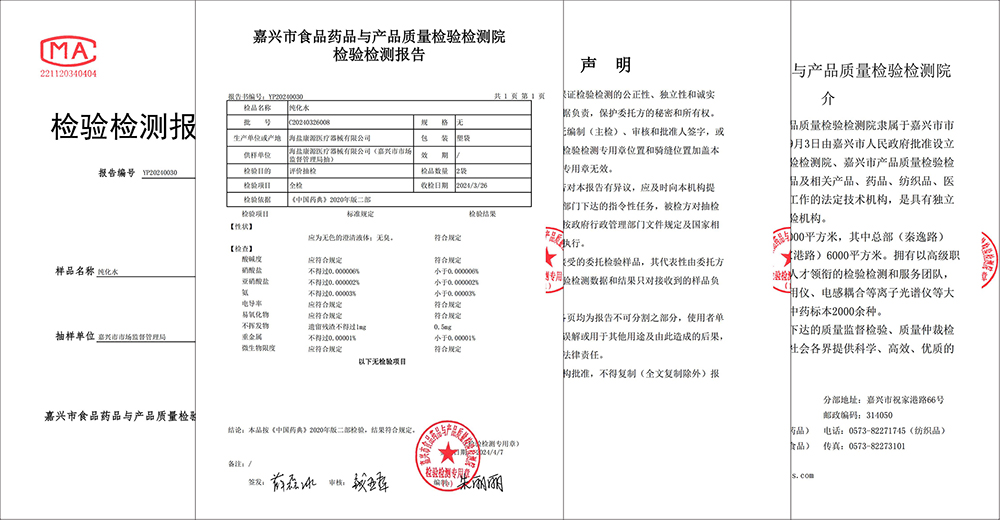
ಕಾಂಗ್ಯುವಾನ್ ಮೆಡಿಕಲ್ ಯಾವಾಗಲೂ ಉತ್ಪನ್ನದ ಗುಣಮಟ್ಟ ಮತ್ತು ರೋಗಿಗಳ ಸುರಕ್ಷತೆಯನ್ನು ಮೊದಲ ಸ್ಥಾನದಲ್ಲಿರಿಸುತ್ತದೆ ಮತ್ತು ಪ್ರಕ್ರಿಯೆ ನೀರಿನ ಗುಣಮಟ್ಟ ನಿಯಂತ್ರಣಕ್ಕೆ ನಿರ್ದಿಷ್ಟ ಪ್ರಾಮುಖ್ಯತೆಯನ್ನು ನೀಡುತ್ತದೆ. ಕಂಪನಿಯು ಸುಧಾರಿತ ನೀರಿನ ಉತ್ಪಾದನಾ ಉಪಕರಣಗಳು ಮತ್ತು ಮೇಲ್ವಿಚಾರಣಾ ತಂತ್ರಜ್ಞಾನವನ್ನು ಪರಿಚಯಿಸಿದೆ ಮತ್ತು ಪ್ರತಿ ಹನಿ ನೀರು ರಾಷ್ಟ್ರೀಯ ಮಾನದಂಡಗಳನ್ನು ಪೂರೈಸುತ್ತದೆ ಎಂದು ಖಚಿತಪಡಿಸಿಕೊಳ್ಳಲು ಉತ್ತಮ ಪ್ರಕ್ರಿಯೆಯ ನೀರು ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆ ಮತ್ತು ಕಾರ್ಯಾಚರಣಾ ಕಾರ್ಯವಿಧಾನಗಳನ್ನು ಸ್ಥಾಪಿಸಿದೆ. ಮಾದರಿ ಪರಿಶೀಲನೆಯ ಅಂಗೀಕಾರವು ಕಾಂಗ್ಯುವಾನ್ ವೈದ್ಯಕೀಯ ಪ್ರಕ್ರಿಯೆಯ ನೀರಿನ ಗುಣಮಟ್ಟದ ದೃಢೀಕರಣ ಮಾತ್ರವಲ್ಲದೆ, ಕಾಂಗ್ಯುವಾನ್ ಮೆಡಿಕಲ್ನ ಒಟ್ಟಾರೆ ಗುಣಮಟ್ಟ ನಿರ್ವಹಣಾ ವ್ಯವಸ್ಥೆಯ ಗುರುತಿಸುವಿಕೆಯಾಗಿದೆ.
ಭವಿಷ್ಯದಲ್ಲಿ, ಕಾಂಗ್ಯುವಾನ್ ಮೆಡಿಕಲ್ ತನ್ನ ಆಳವಾದ ಉದ್ಯಮ ಸಂಗ್ರಹಣೆ ಮತ್ತು ನಿರಂತರ ನಾವೀನ್ಯತೆಯ ಮನೋಭಾವವನ್ನು ಎತ್ತಿಹಿಡಿಯುತ್ತದೆ, ವೈದ್ಯಕೀಯ ಉದ್ಯಮದಲ್ಲಿ ಪ್ರಮುಖ ಪಾತ್ರವನ್ನು ವಹಿಸುವುದನ್ನು ಮುಂದುವರಿಸುತ್ತದೆ, ಉತ್ತಮ ಗುಣಮಟ್ಟದ ಮತ್ತು ಸುರಕ್ಷಿತ ವೈದ್ಯಕೀಯ ಉಪಭೋಗ್ಯ ವಸ್ತುಗಳನ್ನು ಹೊಂದಿರುವ ಹೆಚ್ಚಿನ ರೋಗಿಗಳು ಮಾನವ ಆರೋಗ್ಯದ ಕಾರಣಕ್ಕಾಗಿ ಹೆಚ್ಚಿನ ಕೊಡುಗೆಗಳನ್ನು ನೀಡುತ್ತಾರೆ ಎಂದು ಖಚಿತಪಡಿಸುತ್ತದೆ.
ಪೋಸ್ಟ್ ಸಮಯ: ಮೇ-29-2024

 中文
中文